Necrotizing Enterocolitis (NEC) Registry
The NEC Registry brings the necrotizing enterocolitis community together and collects patient-driven data to advance better care and research.
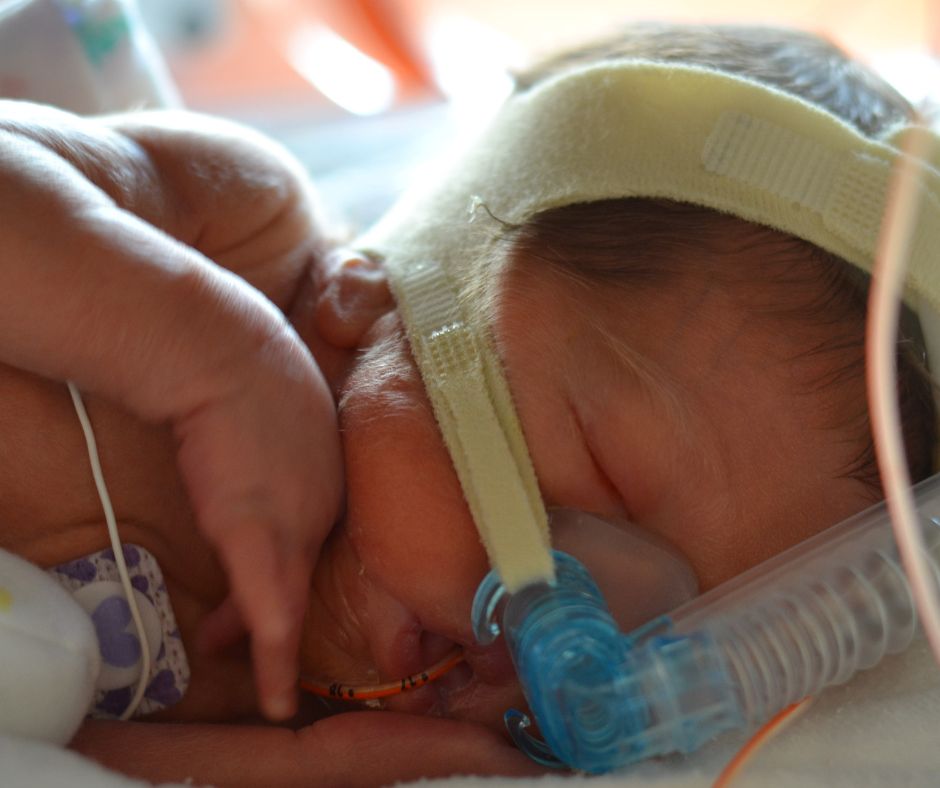
Micah
The NEC Registry Facilitates and Advances Better Care and Research
The NEC Registry is an IRB-approved database built by and for patient-families affected by NEC. The NEC Registry is hosted by the National Organization for Rare Disorders, Inc. (NORD®); an independent non-profit patient advocacy organization dedicated to individuals with rare diseases and the organizations who serve them. NORD is committed to the identification, treatment, and cure of rare disorders through education, advocacy, research, and patient services.
The Registry collects information about patient-families affected by NEC.
How to Participate
Please see the user guide to learn how to join the NEC Registry. If you have questions, please contact us at Registry@NECsociety.org
* Important: The “Participant” is the individual who was diagnosed with NEC, whose information will be entered into the Registry.
What is a patient registry?
A patient registry is a collection of standardized information about a group of patients who share a condition. The information may be used for a variety of purposes such as conducting natural history studies and supporting disease-specific clinical trial recruitment.
Why do we need a NEC Registry?
The impact of NEC on adult survivors and caregivers of infants and children with NEC is poorly understood. NEC is diagnosed during infancy, and the outcomes into childhood, adolescence, and adulthood have not been thoroughly investigated. Most follow-up studies on NEC survivors focus on young ages, and do not fully reflect the lived experiences of patient-families. Longitudinal follow-up studies beyond preschool age will better inform the long-term outcomes and life impacts of NEC.
What is the purpose of the NEC Registry?
The primary aim of the NEC Registry is to conduct a prospectively planned and efficient natural history study that will lead to a better understanding of the disease and its course and pace over time.
Other Registry objectives include:
- Create, promote, and maintain a convenient online platform for adult survivors of NEC and parents/caregivers of children diagnosed with NEC, to self-report the impacts of NEC.
- To develop a contact registry within the NEC Registry (e.g., to notify participants of research studies and clinical trials related to NEC).
- Better characterize the long-term effects of NEC on individuals and families as they relate to physical and mental health, social experiences, and Quality of Life, via patient-reported outcomes.
- To help the NEC community develop recommendations and standards of care.
- To be a case-finding resource for researchers either retrospectively studying the pathophysiology of NEC or intervention outcomes or designing prospective trials of novel treatments.
- Support the design of clinical trials that explore new rare disease treatments;
- Describe the people who have had necrotizing enterocolitis and to better understand the characteristics and variability of those who are diagnosed with necrotizing enterocolitis;
- Understand how social, emotional, and physical outcomes of necrotizing enterocolitis change over a person’s lifetime;
- Learn about clinical practice patterns and variations over the course of treatment;
- Help to develop best practices, management guidelines, and recommendations so that clinicians can know how to give the best care to improve the quality of life and outcomes of people with necrotizing enterocolitis; and
- Identify people who have had necrotizing enterocolitis or families who have a child who has been impacted by NEC who might be willing to take part in other research studies or clinical trials. You will be able to choose whether you want to hear about these other studies.
For Researchers
The NEC Registry collects disease-specific natural history data about individuals with necrotizing enterocolitis, with the goal of improving the understanding of NEC prevention strategies and informing treatment development. Registry questionnaires were built from common data element standards and cover the following topics:
- Patient demographics
- NEC diagnosis and birth hospitalization
- Medical history
- Treatment, diagnosis, and the long-term management of complications
- The quality of life of survivors, parents/caregivers of survivors
- Physical social and emotional impacts of bereaved families

If you would like access to the NEC Registry data for a research project, please contact Erin Pryor, at Registry@NECsociety.org for more information. Access to the NEC Registry data is contingent upon project approval by the NEC Registry Advisory Panel.
Special Acknowledgement
Dr. Allison Rose cared for infants as a neonatologist at Children’s Healthcare of Atlanta. Allison’s leadership within the NEC Society began at the 2019 NEC Symposium, and she became increasingly involved in the NEC Society’s research and advocacy efforts until her devastating cancer diagnosis and passing in 2024. Allison played a pivotal role in establishing the foundation for the NEC Registry. We are forever grateful for Allison’s dedication to patient-families and contributions to our mission of building a world without NEC.
FAQ
Here are some frequently asked questions. If you have additional questions that are not listed, please contact us at Registry@NECsociety.org
How is the registry maintained?
The registry is maintained by NORD, which hosts the registry on its web-based application. NORD provides ongoing technical support of the system. The NEC Society provides the day-to-day management of their patient registry.
The IAMRARE app, which is free to download, gives you a more convenient way to stay involved with our registry, wherever you are. With the app, you can manage your participation, complete surveys, receive updates, and more — all from your phone or tablet.
Download the IAMRARE mobile app today from the Apple App Store and Google Play.
What types of data will be collected in the NEC Registry?
The NEC Registry collects data on the following topics:
- Patient demographics
- NEC diagnosis and birth hospitalization
- Medical history
- Treatment, diagnosis, and the long-term management of complications
- The quality of life of survivors, parents/caregivers of survivors
- Physical social and emotional impacts of bereaved families
Is the data secure?
The NEC Registry follows strict government guidelines to ensure patient information is protected. The platform is served over HTTPS, which means that the data is encrypted when being sent from the user’s browser to the NORD servers. The data is also kept encrypted in the NORD database. Communications between the registry platform application server and the database are also encrypted. As with any information one provides electronically, there is a very rare chance that privacy could be compromised. However, the registry and the security measures minimize the chance of this occurring.
Who can join the study?
This study is open to anyone who was diagnosed with necrotizing enterocolitis and parents/caregivers of a child who was diagnosed with necrotizing enterocolitis and meets the study inclusion criteria for participation.
Individuals who want to enroll in the NEC Registry must be of legal age. A legal adult is defined as a person who is at least 18 years of age and able to consent for themselves or on behalf of a child or other individual in their care.
Individuals must have at least periodic access to the internet and be able to comply with web-based study procedures and data collection.
Is there a cost to participate?
There is no cost to the patient to join this study.
Is there a payment for participating?
You will not be paid for the information you provide.
What is a Research Study Sponsor?
A Research Study Sponsor is an individual, company, institution, or organization. They are responsible for choosing appropriately trained and experienced researchers to conduct the study and for the initiation and management of the research study. Additionally, the sponsor is responsible for the costs associated with conducting a registry study. They ensure that the study is conducted in a reputable, ethical manner and upholds regulations as they apply to the study. The sponsor of this registry is the NEC Society.
Who is the NEC Society?
The NEC Society is the world’s leading nonprofit dedicated to building a world without necrotizing enterocolitis (NEC). The NEC Society brings together clinicians, researchers, and patient-families to better understand, prevent, and treat NEC. The NEC Society was founded in 2014 by Jennifer Canvasser after her son, Micah, died from complications of NEC just before his first birthday. Today, patient-families and experts from around the world work together to improve outcomes for the most vulnerable infants at risk of NEC.
What is a Principal Investigator?
The Principal Investigator (PI) is the person with the primary responsibility for the design and conduct of the research project or study. The PI is responsible for oversight of all aspects pertaining to the conduct of the Registry, its staff and the research on the data contained within.
Who is a Study Participant?
A Study Participant is the individual about whom information is entered into the registry. In the case of an independent person of legal age, this individual will consent for and enter information about themself. If an individual is not of legal age or is an adult who requires someone to act on their behalf, a person (Caregiver/Legally Authorized Representative, see below) who is legally responsible for their health care will provide consent and enter information about the Study Participant.
What is a Legally Authorized Representative (LAR)?
An LAR is someone who is authorized under applicable law to consent and enter data in the registry on behalf of another child or individual who has had necrotizing enterocolitis. The LAR may be a parent, grandparent, spouse, caregiver, or guardian as long as they have the legal authority to grant consent on behalf of that individual.
What is a Designated Representative?
A Designated Representative is a legal adult who was the caretaker of an individual who passed away from necrotizing enterocolitis. This may be a spouse, parent, sibling, offspring, close relative, close friend, guardian and/or significant other of this individual. This person must have had knowledge of and participated in the medical care of the child or individual who has passed away after a NEC diagnosis. These individuals are permitted to enter retrospective data on their behalf.
What is an Informed Consent Form (ICF)?
An ICF is a document that provides potential participants with key information about the registry. This document helps potential participants to make an informed decision whether to join or not. Information will include topics such as: the risks and benefits of the research project, use of data, and participant privacy. If they choose to join the study, participants are required to electronically sign the ICF. This indicates that they agree to the terms as described before entering data into the registry or responding to surveys.
After consenting, can a Participant choose to stop participating in the study?
Participants are able to withdraw from the study at any time. However, researchers may still use the information that they have collected prior to the participant changing their mind. Information that has already been shared with researchers prior to withdrawal cannot be retrieved or removed.
What is an Institutional Review Board (IRB)?
An IRB is a board formally designated by an institution or investigator to review, approve the initiation of, and conduct periodic reviews of research involving people. The primary purpose of such an assessment is to ensure the protection of the rights and welfare of the participants in the study. This is also known as an Ethics Committee (EC) or Research Ethics Board (REB in Canada).
What is a Registry Advisory Panel?
A Registry Advisory Panel is a committee that may include scientists, doctors, and patient advocates. They oversee the conduct of the study. The board advises on the development of surveys and reviews combined registry data and the use of this registry. They will ensure proper evaluation of all research requests for use of the registry data. They will also review any protocol or confidentiality deviations and ensure that any such deviations are reported to the IRB.
How long will this study last?
A registry on the IAMRARE platform will typically be open for at least five years. Participants will be asked to return to the registry periodically to update their information.
Can data be collected worldwide?
The registry uses an online platform that allows participants to contribute data from anywhere in the world. Individuals from other countries who enter data into the registry should be aware that data and privacy laws are different in the U.S. from other countries. This U.S. based registry will protect data and privacy according to U.S. requirements.
What are the GDPR considerations?
For individuals living outside the United States who choose to share information about themselves, the same protections for privacy and confidentiality are offered as in the United States. Residents of the European Union and Switzerland have additional particular rights related to personal information. This information is disclosed within the informed consent document. If an individual signs this document, they acknowledge that they are disclosing information that would otherwise be private. Privacy laws in an individual’s country may have different protections than those provided in the United States.
Registry participants who are residents of the European Union and Switzerland are entitled to:
- Request to obtain access to and rectification or erasure of personal data;
- Receive personal data in a portable, readily-accessible format;
- Restrict or withdraw permission for the processing of personal information; and
- Lodge a complaint with an appropriate supervisory authority.
Where is the data stored?
NORD stores Sponsor and Participant Registry Data on NORD encrypted servers and/or encrypted servers of third-party vendors hosted in Canada. Regular backup at commercially acceptable intervals is provided. These servers meet industry standards and are compliant with US and international regulations, including GDPR.
Who owns the data?
The study data are owned by the study sponsor, the NEC Society. The NEC Society decides how and with whom to share the data by a review of the NEC Registry Advisory Panel. NORD staff will have access to the data for activities related to the support and maintenance of the Platform and will collect Platform-wide participation statistics. The specifics will be outlined in your informed consent.
Who will have access to Protected Health Information (PHI)?
All data, including those with PHI, will be stored in a password-protected secure server. Access to PHI will be limited to:
- Approved members of the NEC Registry research team
- NORD staff, in cases where technical support is needed and with the permission of registry staff
- With agreement from the Sponsor, NORD may conduct IRB-approved, cross-disease research using registry data.
In all cases, your privacy will be protected. The Registry Advisory Board will evaluate all requests for data from researchers. Researchers will only be provided with the minimum data necessary to accomplish their research study goals. Data containing PHI will only be shared if the research cannot be done without it. The researchers will be required to sign a Confidentiality Agreement in which they promise to keep your information safe.
Who is NORD – the National Organization for Rare Disorders, Inc.?
NORD, an independent nonprofit, is leading the fight to improve the lives of rare disease patients and families. We do this by supporting the rare community, its people, and organizations. We work together to accelerate research, raise awareness, provide valuable information, and drive public policy that benefits the estimated 25-30 million Americans impacted by rare diseases.
Learn more about NORD at https://rarediseases.org/

